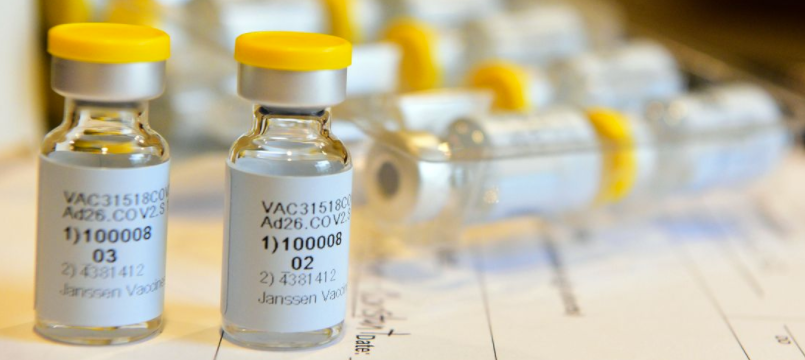
Johnson & Johnson’s experimental Covid-19 vaccine phase 1/2 trial findings have provided some encouragement.
- induced immune responses in most people who received the shot
- displayed an acceptable safety profile
Now for some caveats. These are from a small early-stage trial. They are interim, posted on online preprint server medRxiv. The report is not yet peer-reviewed, not yet published in medical journals.
J&J have said that they’ll now carry on with a larger late-stage study of up to 60,000 people that will provide more definitive evidence.
The link above has more, its the Journal so it may be gated. CNN has an ungated report at this link if you prefer.
- The vaccine — called Ad26.COV2.S — uses the same technology used for Johnson & Johnson’s Ebola, Zika, HIV and RSV vaccines.
This article was originally published by Forexlive.com. Read the original article here.